Specific Heat Capacity
Specific Heat Capacity: Overview
This topic covers concepts, such as, Specific Heat Capacity of Water, Heat Capacity, Specific Heat Capacity, Molar Specific Heat Capacity & Variation of Specific Heat Capacity of Water with Temperature etc.
Important Questions on Specific Heat Capacity
The molar heat capacity of water at constant pressure is . When 1kJ of heat is supplied to 100g of water, which is free to expand, the increase in temperature of water is
Two different liquids of same mass are kept in two identical vessel, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperaure vs time plot for the two materials. We denote the specific heat in the liquid states to be and for materials and respectively, and latent heats of fusion and respectively.
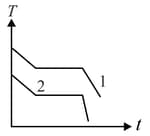
Choose the correct option.
200 g water is heated from to . Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water J/kg/K):
Water is used as a coolant because
A person weighing takes in diet per day. If this energy were to be used in heating the body of person without any losses, then the rise in his temperature is (specific heat of human body )
Amongst object A and B, if the specific heat of object A is less than of object B, then
The amount of heat required to raise the temperature of mass of water through is called its
The amount of heat required to raise the temperature of mass of water through is called its
A lead bullet strikes a target with a velocity of After the impact, the bullet falls dead and the heat produced in the process gets equally shared between the bullet and the target. If the specific heat capacity of lead is then the rise in temperature of the bullet is (Assume that kinetic energy of a bullet is totally converted into heat).
Specific heat of a substance is given by , find out amount of heat required to raise the temperature of substance from to
The number of degrees of freedom of a gas whose specific heat capacity at constant pressure is is
(universal gas constant )
The amount of heat required to raise the temperature of liquid from to if its specific heat varies with temperature as (where is temperature in ) is
of steam at is passed into a large block of ice at the mass of ice that melts is
ice at is mixed with steam at . The minimum value of so that finally all ice and steam converts into water is,
(Use, , (melting) and (vaporization).)
Which one of the following would raise the temperature of of water at most, when mixed with:
Which statement is false for specific heat of water?
Two identical bodies are made of a material for which the heat capacity decreases with increase in temperature. One of these is at while the other one is at If the two bodies are brought into contact, then the final common temperature will be-
A mixture of moles of monoatomic gas and moles of diatomic gas has . Then:
A tap supplies water at . A man takes of water per minute at from a geyser connected to the tap. The power of geyser is...(Take )
The thermal capacity of of aluminium (specific heat ), is
